- Topic1/3
11k Popularity
30k Popularity
14k Popularity
5k Popularity
172k Popularity
- Pin
- Hey fam—did you join yesterday’s [Show Your Alpha Points] event? Still not sure how to post your screenshot? No worries, here’s a super easy guide to help you win your share of the $200 mystery box prize!
📸 posting guide:
1️⃣ Open app and tap your [Avatar] on the homepage
2️⃣ Go to [Alpha Points] in the sidebar
3️⃣ You’ll see your latest points and airdrop status on this page!
👇 Step-by-step images attached—save it for later so you can post anytime!
🎁 Post your screenshot now with #ShowMyAlphaPoints# for a chance to win a share of $200 in prizes!
⚡ Airdrop reminder: Gate Alpha ES airdrop is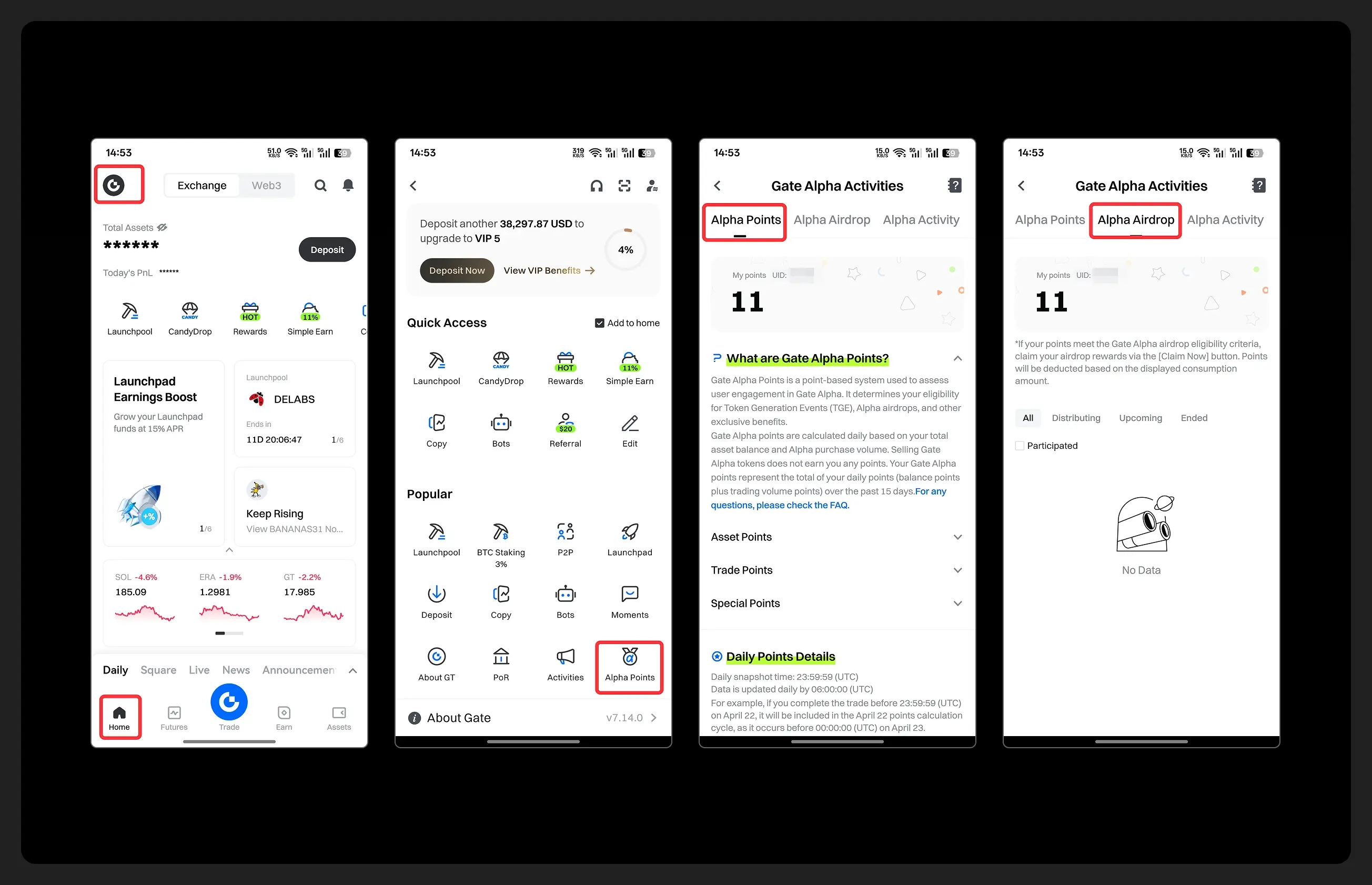
- Gate Futures Trading Incentive Program is Live! Zero Barries to Share 50,000 ERA
Start trading and earn rewards — the more you trade, the more you earn!
New users enjoy a 20% bonus!
Join now:https://www.gate.com/campaigns/1692?pid=X&ch=NGhnNGTf
Event details: https://www.gate.com/announcements/article/46429
- Hey Square fam! How many Alpha points have you racked up lately?
Did you get your airdrop? We’ve also got extra perks for you on Gate Square!
🎁 Show off your Alpha points gains, and you’ll get a shot at a $200U Mystery Box reward!
🥇 1 user with the highest points screenshot → $100U Mystery Box
✨ Top 5 sharers with quality posts → $20U Mystery Box each
📍【How to Join】
1️⃣ Make a post with the hashtag #ShowMyAlphaPoints#
2️⃣ Share a screenshot of your Alpha points, plus a one-liner: “I earned ____ with Gate Alpha. So worth it!”
👉 Bonus: Share your tips for earning points, redemption experienc
- 🎉 The #CandyDrop Futures Challenge is live — join now to share a 6 BTC prize pool!
📢 Post your futures trading experience on Gate Square with the event hashtag — $25 × 20 rewards are waiting!
🎁 $500 in futures trial vouchers up for grabs — 20 standout posts will win!
📅 Event Period: August 1, 2025, 15:00 – August 15, 2025, 19:00 (UTC+8)
👉 Event Link: https://www.gate.com/candy-drop/detail/BTC-98
Dare to trade. Dare to win.
The first three common De-Da-Botu monoclonal antibody has been approved in Japan for the treatment of breast cancer.
On December 28, Jinshi Data reported that on December 27, local time, The First Three Company announced that the targeted TROP2 antibody-drug conjugate (ADC) DATROWAY® (trastuzumab deruxtecan) developed by the company in collaboration with AstraZeneca has been approved in Japan for the treatment of adult patients with unresectable or recurrent breast cancer who are hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-), and have an immunohistochemistry (IHC) score of 0, 1+, or 2+ with in situ hybridization (ISH-) after receiving chemotherapy.